First Revenues Generated Upon Launch of Satipharm.com
First Revenues Generated Upon Launch of Satipharm.com
Perth, Aug 31, 2015 AEST (ABN Newswire) - MMJ PhytoTech Limited ( ASX:MMJ) is pleased to announce that its Swiss-based subsidiary Satipharm generated the Company's first revenues from sales of its game-changing CBD (Cannabidiol) Capsules in the month of August. The Company also announces the official launch of its online direct sales platform, Satipharm.com, where retail customers in the European Union (the "EU") can purchase the Capsules today. Tomas Edvinsson, CEO of Satipharm commented:
ASX:MMJ) is pleased to announce that its Swiss-based subsidiary Satipharm generated the Company's first revenues from sales of its game-changing CBD (Cannabidiol) Capsules in the month of August. The Company also announces the official launch of its online direct sales platform, Satipharm.com, where retail customers in the European Union (the "EU") can purchase the Capsules today. Tomas Edvinsson, CEO of Satipharm commented:
"MMJ is very happy with this landmark accomplishment and we leave the month of August with significant momentum, on track to achieving all stated milestones."
Highlights:
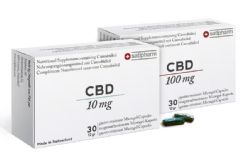
- Satipharm CBD Gelpell(R) Gastro-Resistant Microgel Capsules ("Capsules") are now available to retail and wholesale customers
- Capsules are the first product offered on newly-launched online direct sales platform, Satipharm.com, available to retail customers in 30x10mg and 30x100mg blister packs for EUR89 and EUR579, respectively
- Wholesale strategy kicked-off early August offering bulk or blister packs under Satipharm or private label branding, with a few sizeable deals currently being negotiated
- Capsules are the first product launched by Satipharm under an exclusive development agreement with Swiss partners Ai Fame GmbH and Gelpell AG
- The 100% Swiss-made Capsules contain pharmaceutical grade, GMP-produced mono-compound CBD derived from GACP cultivated medicinal hemp and utilise proprietary delivery technology which greatly enhances bioavailability
- Laura Boersen, MBA, biochemist and professional pharmaceutical representative with significant European experience, appointed Head of International Sales
- First revenues have already been generated from sales of the Capsules, driving the strategy to become a self-funded independent drug development company
Game-Changing CBD Capsule
The proprietary, GMP-produced Satipharm CBD Gelpell(R) Gastro-Resistant Microgel Capsules consist of pharmaceutical-grade GACP/GMP produced CBD mono-compound and are registered as a dietary supplement in Germany. The Capsules are produced in partnership with Ai Fame GmbH, an integrated cannabis-focused pharmaceutical compound manufacturer and Gelpell AG, a premium contract manufacturer of supplements and Phytopharmaka, both based in St. Gallen, Switzerland.
The active ingredient Cannabidiol (CBD) is derived from a proprietary medicinal cannabis strain that is grown in Switzerland under controlled GACP standards and extracted by Ai Fame GmbH under GMP protocols. The Capsules are entirely produced in Switzerland under supervision of the Swiss Health Authorities. Further, the Capsules utilise Gelpell's proprietary Microgel capsules, which ensure accurate and consistent dosages and substantially enhance the bioavailability of the active ingredients through its unique, controlled delivery technology.
The 100% Swiss-made, GMP-produced Capsules are set to be a game-changer in the EU CBD market. Competing CBD products tend to be sold as crude liquid extracts and oils derived from field-grown, non-quality controlled industrial hemp, and often have issues with consistency, dosage and quality. Despite the Capsules' superior quality the Company has priced them in line with competing CBD products to support maximum market penetration.
MMJ Becomes a Revenue Generating Company
MMJ became a revenue generating company in the month of August with the first sales of Capsules from its new online direct sales platform Satipharm.com. This is a pivotal point for the Company and its shareholders as it represents the first step towards MMJ's strategic goal of becoming a self-funded independent drug development company.
The Company has seen strong interest from retail customers since the launch of Satipharm.com with initial orders flowing in prior to any marketing or announcement of the website. This has created an immediate source of revenue for MMJ. Additionally, the level of interest from potential wholesale customers is much higher than expected. MMJ is encouraged by this strong initial demand and looks forward to the coming months as this revenue source grows.
The Company intends to produce a total of 1,000,000 capsules in 2015, selling at approximately EUR3 per capsule retail and EUR1.95 wholesale.
Focused Sales and Marketing Strategy
The Capsules are set to be a potential leader in the highly fragmented EU CBD supplement market which is characterised by intermittently available, low quality and low purity products of questionable origin. To the Company's knowledge, the Capsules will be one of the first CBD supplements containing GACP cultivated ingredients produced to GMP-standards registered in a EU member country for sale as a supplement. The capsules are further differentiated by the proprietary Gelpell(R) Gastro-Resistant Microgel delivery technology which greatly enhances the bioavailability of the CBD. Given that the Capsules are priced in line with the existing available inferior products, Management anticipates gaining meaningful market share in the near to mid-term.
This is supported by initial indications from the retail and wholesale sales strategies for the Capsules recently kicked-off by the Company. Demand from retail consumers has been strong and there is currently a higher level of interest from wholesale customers than expected, with the Company currently negotiating deals with a few wholesale customers which was not anticipated at this stage.
The retail sales strategy consists of driving sales of the Capsules through the Company's newly launched online direct sales platform, Satipharm.com. A moderately sized digital marketing campaign is planned to support the launch of the pill over the next few months after which point a longer-term digital campaign will be designed. Additionally, the Company is implementing a media strategy aimed at encouraging organic coverage of the Capsule in print and online which will be supported by strategically placed sponsored content. The Company also believes that word of mouth recommendations will account for a meaningful amount of demand. The Capsules are sold to retail customers under the Satipharm brand in 30x10mg and 30x100mg blister packages that retail for EUR89 and EUR579, respectively.
For wholesale customers, the Capsules are available in blister packs or in bulk and can be delivered under the Satipharm brand or with private labeling. The wholesale sales strategy consists of targeting supplement and nutraceutical marketers and retailers under both custom private labelling and Satipharm branding. Together, the Company and its partners have sufficient contacts in the targeted space to implement the strategy as it is currently envisaged. Although the margins are considerably lower when selling into the wholesale space, the long-term potential for high sales volume in a wholesale dominated sales strategy more than offsets the lower margins. Given initial limited supply of the Capsules the Company may investigate exclusive supply deals or similar strategies to combat loss in margins.
Leading these initiatives is newly appointed Satipharm Head of International Sales Laura Boersen, MBA. Mrs. Boersen is a biochemist/microbiologist and professional pharmaceutical representative with significant European experience.
About Good Manufacturing Practice (GMP) and Good Agricultural Production and Collection Practice (GACP)
The active compound of the Capsules is produced by Ai Fame under GMP protocols, which are a set of practices applicable to the pharmaceutical manufacturing sector that conform with quality and safety regulations issued by the Food and Drug Administration (FDA), World Health Organisation (WHO) and other global health agencies and regulations.
The raw materials for the extraction are grown under Good Agricultural and Collection Practices (GACP) for medicinal plants as developed by the WHO. GACP provides general technical guidance on obtaining medicinal plant materials of good quality for the sustainable production of herbal products classified as medicines. GACP guidelines are concerned with the cultivation and collection of medicinal plants and include post-harvest operations.
CBD and its Therapeutic Potential
Cannabinoids are a class of chemical compounds - including tetrahydrocannabinol (THC) and cannabidoil (CBD) - contained in the cannabis plant. CBD is the non-psychoactive cannabinoid that has reported significant positive health benefits without the intoxicating effect of THC. Importantly, the capsules do not contain THC.
Scientific research has shown that CBD may have positive effects on many conditions, including chronic pain, cancer, anxiety, diabetes, epilepsy, rheumatoid arthritis, PTSD, sleep disorders, cardiovascular disease, antibiotic-resistant infections, and various neurological ailments.
Whilst research in the medical cannabis industry is still at a very early stage, there are clear indications that CBD has positive impacts on a wide range of conditions and is now being recognised by governments, research institutes, pharmaceutical companies and other organisations worldwide as having real medicinal potential.
As developments in the industry continue MMJ is well positioned to become a leading provider of CBD based supplements.
Andreas Gedeon, CEO and Managing Director of MMJ commented:
"August was a month of 'firsts' for the Company - it was our first month as the newly merged MMJ PhytoTech Group, our first clinical study was approved, the first CBD Capsules were available on our new online direct sales platform and the Company's first revenues were generated. These achievements represent the progress we have made as a company in the implementation of our 'Farm to Pharma' strategy that will see our operations spanning the entire medical cannabis value chain."
"The limited supply of such products in the market supports robust prices for our CBD capsules and, based on initial demand indications, we're confident of achieving significant revenues from this capsules and other products we intend to bring on-line in the near future."
About MMJ Group Holdings Ltd

MMJ Group Holdings Ltd (ASX:MMJ) is a global cannabis investment company. MMJ owns a portfolio of minority investments and aims to invest across the full range of emerging cannabis-related sectors including healthcare, technology, infrastructure, logistics, processing, cultivation, equipment and retail. For MMJ's latest investor presentation and news, please visit: https://www.mmjphytotech.com.au/investors/


![abnnewswire.com]()
Related Companies
Social Media
Share this Article

 ASX:MMJ) is pleased to announce that its Swiss-based subsidiary Satipharm generated the Company's first revenues from sales of its game-changing CBD (Cannabidiol) Capsules in the month of August. The Company also announces the official launch of its online direct sales platform, Satipharm.com, where retail customers in the European Union (the "EU") can purchase the Capsules today. Tomas Edvinsson, CEO of Satipharm commented:
ASX:MMJ) is pleased to announce that its Swiss-based subsidiary Satipharm generated the Company's first revenues from sales of its game-changing CBD (Cannabidiol) Capsules in the month of August. The Company also announces the official launch of its online direct sales platform, Satipharm.com, where retail customers in the European Union (the "EU") can purchase the Capsules today. Tomas Edvinsson, CEO of Satipharm commented: