FDA 510(k) approval for BARM(TM) version 1 and Appointment of Chief Scientific Officer
Cortical secures USA 510(k)
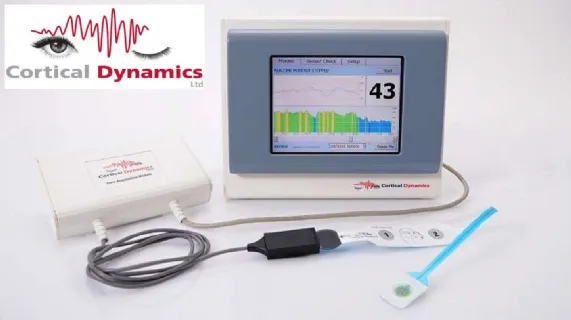
Perth, Sep 4, 2023 AEST (ABN Newswire) - BPH Energy Limited ( ASX:BPH) is pleased to announce that its investee company Cortical Dynamics Limited ("Cortical" or "the Company") has secured FDA 510(k) clearance in the USA for its flagship technology, the Brain Anaesthesia Response Monitor or BARM(TM) system version 1.
ASX:BPH) is pleased to announce that its investee company Cortical Dynamics Limited ("Cortical" or "the Company") has secured FDA 510(k) clearance in the USA for its flagship technology, the Brain Anaesthesia Response Monitor or BARM(TM) system version 1.
Highlights
- Cortical has secured FDA 510(k) clearance in the USA.
- 510 (k) clearance by the FDA is a major milestone in the development of the Company
- FDA clearance lays the foundation for the commercialisation of the BARM(TM) system in the USA.
- Appointment of Chief Scientist
The clearance is a result of two years' work post submission with the US Food and Drug Administration (FDA) in 2021, Cortical being ably assisted by MCRA, a leading Washington based medical global full-service medical device, diagnostics, and biologics CRO and consulting advisory firm.
The 510(k) clearance for BARM(TM) version 1 in the USA is complemented by existing regulatory approvals in Australia (TGA), Europe (CE) and South Korea (KMFDS).
The BARM(TM) Pec "plug and play "version 1 was approved compatible by Philips with its IntelliView operating room monitors earlier this year.
Cortical is working on an enhanced version of BARM(TM) with its partner AIT (the Austrian Institute of Technology) based in Vienna which will include upgrades to the software, hardware and firmware.
BPH and Cortical Director, Mr David Breeze stated that "the 510 (k) clearance by the FDA is a major milestone in the development of the Company which lays the foundation for the commercialisation of the BARM(TM) system in the USA".
Appointment of Chief Scientist
Additionally, building on the technical and regulatory developments in the Company Cortical has recently appointed Dr Sunil Nagaraj PhD as its new Chief Scientist.
Dr. Sunil Belur Nagaraj obtained his Master's Degree from the University of Victoria in Canada in 2010; and Doctoral Degree from University College Cork, Ireland in 2015. His doctoral research centered around the development of AI-based real-time brain monitoring, utilising EEG recordings to monitor brain activity. After a role as a postdoctoral fellow at the Harvard Medical School/Massachusetts General Hospital in the USA. Dr Nagaraj assumed the position of an Assistant Professor of medicine in the at the University Medical Centre Groningen in The Netherlands for two years. Concurrently, he dedicated three years to working as a scientist at Royal Philips, where he specialised in sleep disorders at the Innovation Forum, highlighting its potential to provide future insights into heart-brain connectivity.
Throughout his career, Dr. Nagaraj has demonstrated exceptional research acumen, with a patent and 21 high-impact journal articles to his name, amassing over 650 pioneering research papers and has been recognised through several national and international grants, enabling him to conduct cutting-edge studies that contribute significantly to the advancement of medical technology.
About Cortical
Neurotechnology Company Cortical Dynamics has developed the BARM(TM) (Brain Anaesthesia Response Monitor) a next generation certified class II personalised Depth of Anaesthesia Monitoring Device for use in the operating room and ICU , complemented by CORDYAN(TM) (Cortical Dynamics Analytics) a proprietary deep learning system/App focusing on anaesthesiology.
Cortical has met the necessary prerequisites of the Philips License and Cooperation Agreement to claim the BARM-PEC as a compatible and "supported device" for Philips Patient Monitoring Systems IntelliVue MP40-90 and MX400-850 using the Philips IntelliBridge EC10 Interface Module or IntelliBridge EC10 integral Interface Board, as well as Philips IntelliBridge System Release C.0 and Patient Information Center iX using the EC40/80 Hub with Open Interface driver (ED/BD101) and EC5 ID Module #106.
About BPH Energy Limited
 BPH Energy Limited (ASX:BPH) is an Australian Securities Exchange listed company developing biomedical research and technologies within Australian Universities and Hospital Institutes.
BPH Energy Limited (ASX:BPH) is an Australian Securities Exchange listed company developing biomedical research and technologies within Australian Universities and Hospital Institutes.
The company provides early stage funding, project management and commercialisation strategies for a direct collaboration, a spin out company or to secure a license.
BPH provides funding for commercial strategies for proof of concept, research and product development, whilst the institutional partner provides infrastructure and the core scientific expertise.
BPH currently partners with several academic institutions including The Harry Perkins Institute for Medical Research and Swinburne University of Technology (SUT).
| ||
|